GU-PASS Clinical Trial Module
New Clinical Trial Contracts for Study Coordinators
Go to the GU-Pass website and login with your NetID and NetID password.
Click on the New Clinical Trial Contract box.

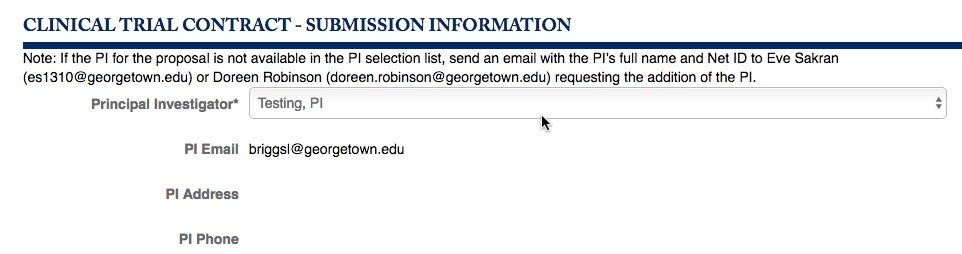
You will be lead to the Clinical Trial Contract – Submission Information section. Choose your Principal Investigator from the pull-down menu.
If you do not see the specific Investigator you need, write to GU-PASS, Eve Sakran or Doreen Robinson.
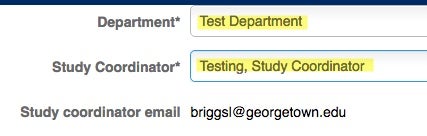
Choose the Department and the Study Coordinator’s information. This is where your information (email address, physical address and department) should be listed.
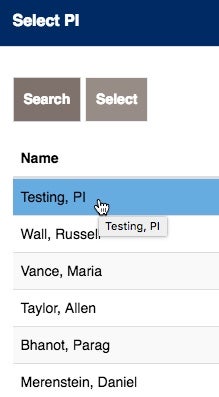
There may be times that the Study Coordinator needs to list other Georgetown University or Georgetown University Hospital Investigators.
If you see their name, click on ADD.

Choose the additional Investigator and add them, by choosing select.
Upload the Confidentiality Disclosure Agreement (one should be available from your computer). Attach the completed form to properly add to this submission.

Once you have attached the proper document, you will see it listed below.

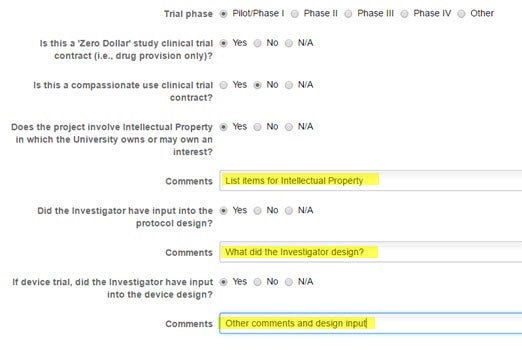
Identify the Phase, whether this is a ‘zero dollar’ trial, if the intellectual property is involved, and particulars about the design.
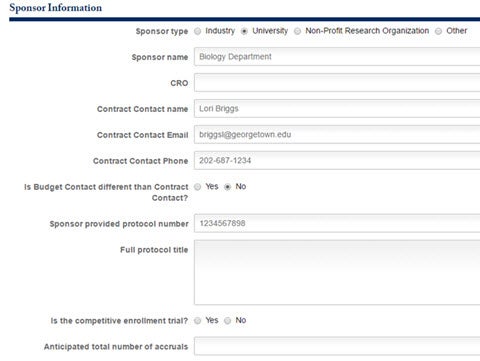
The section following is for Sponsor Information. Specify the Sponsor Type, Name, CRO, and Contact Information.
Transmittal Data is the section that identifies where the study will take place. Answering the questions that follow may require
additional information. If you were to say ‘yes’ to the project involving additional sites, another box would appear for you to
identify those sites.

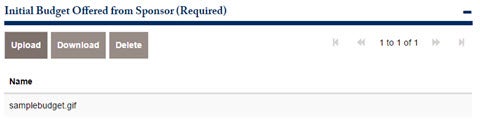
There are 3 required attachments for this application to move to the next approval. The first is the Initial Budget offered from
Sponsor. Attach the document below.
If there are additional documents that need to be attached, please attach them below.

If you have forms available, upload them. In addition, the IRB Protocol numbers should be listed, along with the submitted
and approval date. There are calendars at the end of the IRB submitted/approved dates to assist you with the dates you are
adding.

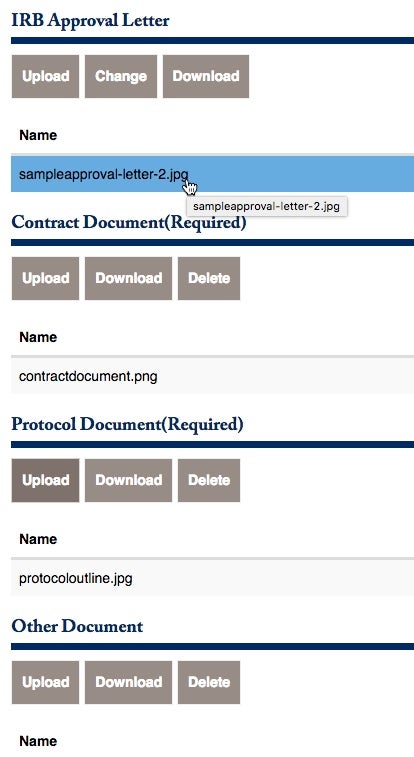
There are additional forms that should be uploaded. Please pay attention to the required documents that must be properly uploaded.
They are (1) The Initial Budget Offered from Sponsor, (2) The Contract Document, and (3) the Protocol Document.
When you save the contract, you will see the formal number (ID) as well as the status. This example has an ID of 16206.

Now that you have the information necessary, click to Submit for PI Review.

You will be prompted to confirm. Choose to proceed.
The document has been moved to the PI’s queue. If the PI has concerns about the contract, they can submit the information back to you.
Login with your NetID and NetID password, and you will see within the status that there are changes that need to be made. Click on
View/Edit/Act in the upper-left corner.

You will see the notes from the PI listed below.

Resubmit your contract.

Confirm and Proceed.
With the changes made, this moves back to the PI for another approval. In addition, there may be situations when the Department Chair will send a contract for your review. You will receive a contract with an explanation of the changes that need to be made. Click to
add comments, and make the necessary changes. Finally, Re-Submit.

The notes have been placed for the Department Head to see. Re-submit and confirm.
New Clinical Trial Contracts for PI – Principal Investigators
The PI should receive an email explaining that there is a contract that has been placed in your queue, and we are waiting for you to review it. Go to: the GU-PASS website and login with your University NetID
and NetID password. Click on the View/Edit/Act button to move to the next steps in Contract Review.

You will be prompted to review the information, and click Yes or No.

If you were to choose ‘no’, you would need to add information to explain to the Study Coordinator what needs to be added or changed. Click on New.

A box to add your notes will open. Please provide any information to assist the Study Coordinator with their documentation. Click on save when your notes are complete.
As soon as you complete your notes, click to send them back to the Submitter.

Confirm that this is your intention. Click on Proceed.
You will receive an email once the requested changes have been made. This is to inform you that the contract is back in your queue. Login. You will notice
that your status says ‘PI Review’. Click on View/Edit/Act in the upper-left corner.
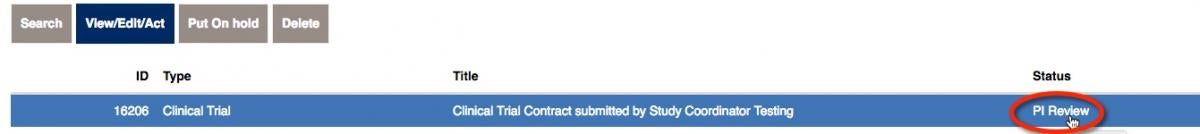
Upon review, all of the information should be correct. Choose “yes” to approve the contract. On the right side, choose to Submit for Department Review.

Choose to Proceed.
The contract moves on to the Department Head. The Department Head will receive an email prompting them that a contract is awaiting their approval.
New Clinical Trial Contracts for Department Heads
The Department Head should receive an email explaining that there is a contract that has been placed in your queue. Please review and approve. Go to the GU-Pass website and login with your NetID and NetID password.
Click on the items within your review queue. You will notice that the status of the item below is “Department Review”. Click on the View/Edit/Act button to move to the next steps.
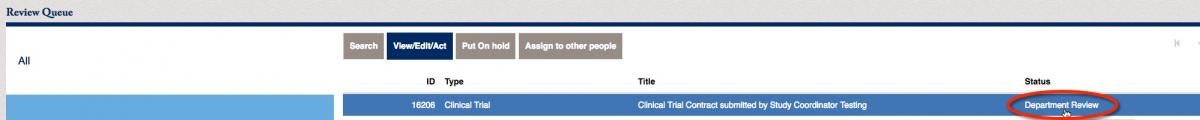
You will be prompted to review the information, and click Yes or No. If you choose to send it back, click on No. You can add comments to the button below, then Send Back to Submitter on the right side.
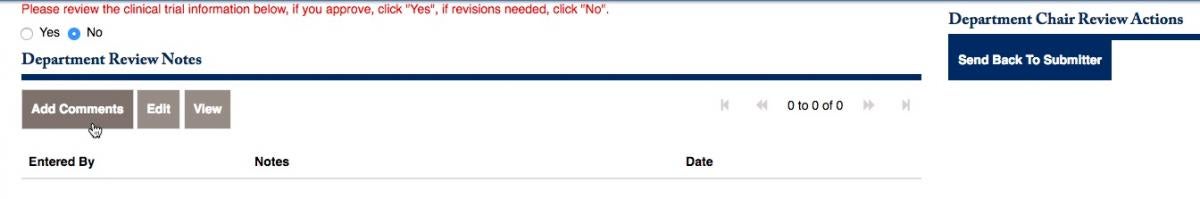
Once you have added the notes that will be sent back to the PI, choose to Send Back to the Sumitter, and click on Proceed.
Once the Study Coordinator has made the necessary changes, the Department Head will receive another email message. Log back into GU-PASS. The status will say up for Department Review (right side).

Upon review, the Department Head is ready to approve. Choose Yes and Submit Department Chair Review (right side).
Confirm and Proceed.